Specific Heat Capacity Units
It also includes below conversion tables. Experiment and Calculation of Reinforced Concrete at Elevated Temperatures 2011.
Chem 101 General Chemistry Topic
Heat capacity ratio of heat absorbed by a material to the temperature change.

. The amount of heat supplied to heat an object can be expressed as. Share calculation and page on. Kelvin joule Units JkgK.
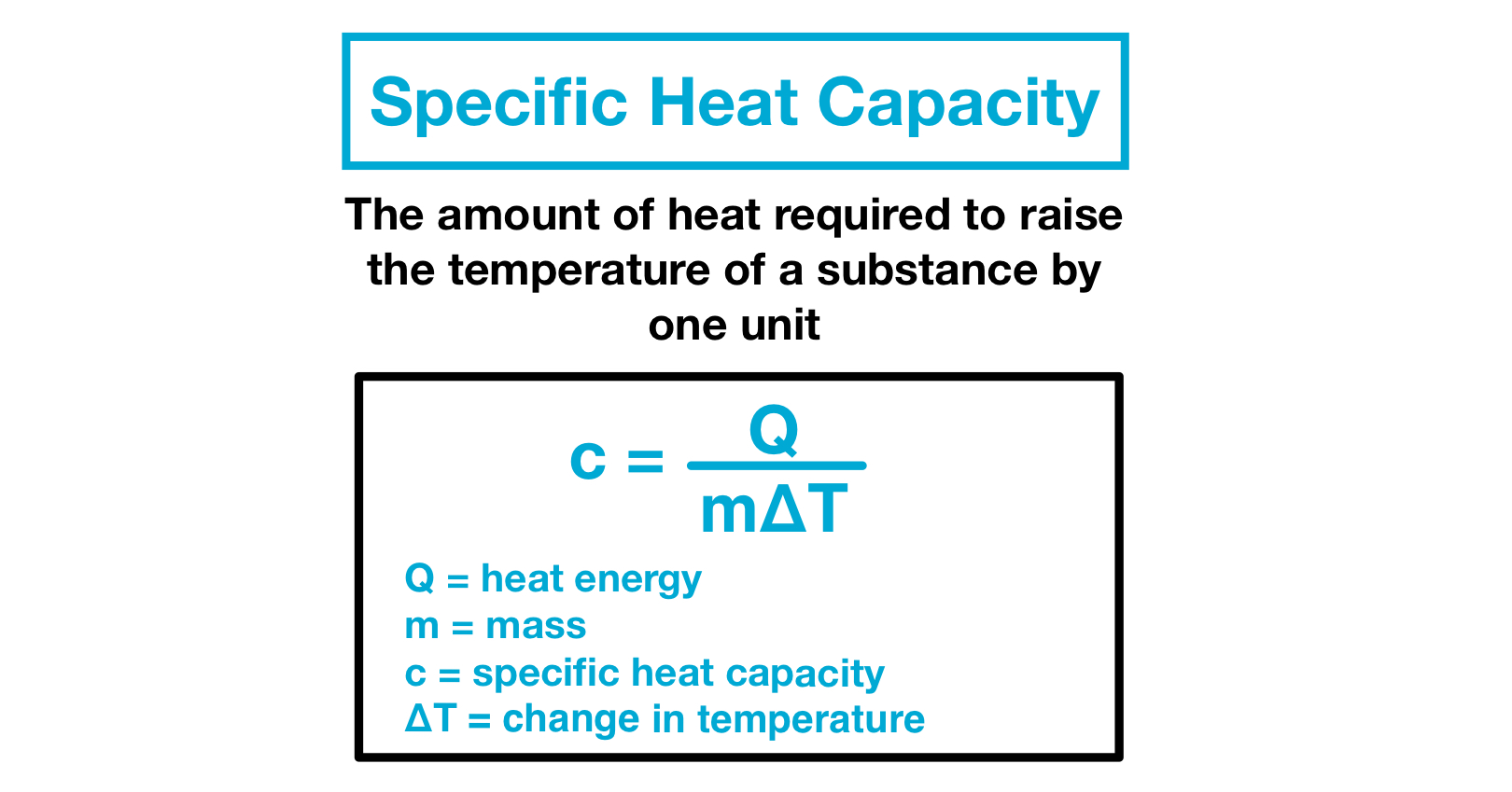
The heat needed to raise a substances temperature by 1 degree Celcius is called the specific heat capacity. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. Free online specific heat capacity converter - converts between 20 units of specific heat capacity including joulekilogramK JkgK joulekilogramC JkgC joulegramC JgC kilojoulekilogramK etc.
Hence its derived SI unit is J kg1 K1. For example the specific heat of water is 1 calorie or 4186 joules per gram per Celsius degree. Specific heat the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree.
Thus the correct option is A. Heat capacity is measured in SI units and is referred to as the joules per kelvin JK unit. The Scottish scientist Joseph Black in the 18th century noticed that equal masses of.
Definition of Specific heat capacity revealed that it is the amount of heat required to increase the temperature of 1 kilogram of any substance by 1 kelvin. No duct work is needed. Joule per kg per Kelvin.
Specific heat can be defined as the amount of heat required to raise the temperature of 1 gram of a substance by 1 degree celsius. Sound Specific Activity Specific Area Specific Cake Resistance Specific Energy Specific Entropy Specific Fuel Consumption Specific Heat Capacity Specific Humidity Specific Volume Specific Weight Speed Stiffness Constant. The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity.
The specific heat capacity is defined as the quantity of heat J absorbed per unit mass kg of the material when its temperature increases 1 K or 1 C and its units are Jkg K or Jkg C. A specific heat capacity calculator is functioned to deliver the outcomes along with standardized units. Units of Heat - BTU Calorie and Joule - The most common units of heat BTU - British Thermal Unit Calorie and Joule.
Q amount of heat supplied J Btu. Calorie or Joule g specific. List of thermal conductivities Note that the especially high molar values as for paraffin gasoline water and ammonia result from calculating specific heats in terms of moles of molecules.
Specific heat capacity is measured in Jkg K or Jkg C. If specific heat is expressed per mole of atoms for these substances none of the constant-volume values exceed to any large extent the theoretical. Specific heat capacity Converter.
Table of Specific Heat Capacities. When you find the heat capacity of one unit of something 1 gram 1 ounce 1 kilogram etc youve found this objects specific heat. Also explore many other unit converters or learn more about specific heat capacity unit conversions.
For example it takes 417 Joules to raise. Water - Specific Heat vs. The heat capacity in calories per gram is called specific heat.
Instead a head unit or multiple head units traditionally mounted on the wall are installed inside with an accompanying unit outside. Know that specific heat refers to the energy needed to raise one gram by one degree. Specific Heat Capacity Unit.
Q C dt 1 where. Unit converter converts the different units of measurement for the same quantity through multiplicative conversion factors. The SI unit of heat capacity is joule per kelvin JK.
Ductless heat pumps or minimulti split heat pumps are an increasingly popular alternative to radiator or baseboard heating as well as replacement for window units for cooling. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. This calculator converts Specific heat capacity units.
The definition of the calorie is based on the specific heat of water defined. Temperature - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F - SI and Imperial units. Specific Heat Capacity c is the amount of heat required to change the temperature of a mass unit of a substance by one degree.
The constant pressure of the specific heat capacity of steam is 18723 kJkg K. The heat capacity of a substance commonly abbreviated as thermal capacity capital C is a measure of the amount of heat needed to change the temperature of the substance by a specific amount. Heat capacity is an extensive propertyThe corresponding intensive property is the specific heat capacity found by dividing the heat capacity of an object.
The units to express specific heat capacity are calK cal JK and J. Heat Capacity has the units of energy per degree. What are the units for specific heat capacity.
Heat mass specific heat capacity change in temperature. The units of specific heat are usually calories or joules per gram per Celsius degree. The specific heat power of water is 42 joules per gram per Celsius degree.
Mass Thermal Capacity Units. Specific heat tells you the amount of energy needed to raise each unit one degree. The formula is.
The expression for specific heat has been. The constant volume of the specific heat capacity of steam is 14108 kJkg K. The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 C.
Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin.
Heat Capacity Of Water Overview Importance Expii

Temperature Thermodynamics Units In Calculations Of Heat Q Physics Stack Exchange

Physics Ch 0 5 Standard Units 12 Of 41 Specific Heat And Molar Heat Capacity Youtube

No comments for "Specific Heat Capacity Units"
Post a Comment